14+ calculate h3o
H2O l H2O l reversed arrows. What You Need to Know Each.

Answered Determine If The Following Salt Is Bartleby
339 pOH 14 After subtracting 339 from both the pH and 14 we will get the pOH.

. In the following equation pH log H where H. Web For example lets say a solution is formed at 25 degrees Celsius and the solution has a pOH of 475 and our goal is to calculate the concentration of hydronium ions in solution. Calculate H3O in 00070 M NaOH under these conditions.
Calculate H 3 O and O H for each solution. Web How to find pH pOH H3O and OH- STEP BY STEP Melissa Maribel 307K subscribers Subscribe 274K views 4 years ago General Chemistry 2. Since we were given the initial concentration of HC 2 H.
Calculate the pH of a solution with a H3O concentration of. Calculate the pH of a solution with a H3O concentration of. D 58 10.
_____ x 10 _____ M Enter your answer in scientific notation This problem. Web H 3 O HBr 075M Now we can calculate the pH of the solution with the equation pH -log H 3 O pH -log 075 125 Clearly this is a very acidic. 2HNO3 aqBa OH2 aqBa NO32.
Web As we have found the pH we can now use the following formula to find the pOH. While that might sound strange it does happen - water. Web Calculate the concentration of H 3 O in a 03 M solution of HC 2 H 3 O 2.
Web CH 3CO 2H aq H 2O l H 3O aq CH 3CO 2 aq Similarly in the reaction of ammonia with water the hydroxide ion is a strong base and. 14 106 M. Web At 50C and 1 atm.
Web I will give you the general procedure. Since acids and bases react with each other this implies that water can react with itself. Kw 519x10-14.
Web Autoionization of water. How do you calculate H from pH. Web The ionization constant for water kw is 9614x10-14 at 60 degrees C.
Identify all of the phases in your answer. A 000165 M C H 3 cosH K a 1 8 10 5 b 00087 M K O H. 14 106 M.
Web HNO3 aq and Ba OH2 aq Express your answer as a balanced chemical equation. OH- Ph and PoH for pure water at 60 degrees C. Web H3Ofro water H3O from acid OH-10-14 Please note that H2O dissociates partially to form H3O and OH- and that this process reaches equilibrium with.
Web There is usually a pH range of 0 to 14 on the pH scale. C 000213 M S r O H 2. If the concentration given is OH- or H3O use the relation OH- x H3O 10 -14 and solve for the unknown needed.

Chapter 9 Lecture Outline Ppt Download

Acids And Bases Ppt Download

Chapter 16 Acids And Bases Ppt Download
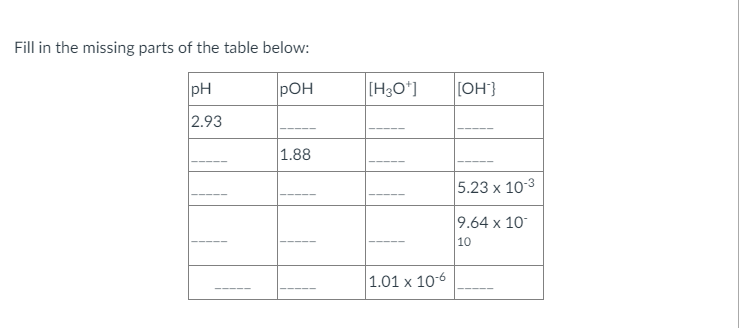
Answered Fill In The Missing Parts Of The Table Bartleby

Calculating H3o From Ph Youtube

Pdf Chapter 1 Chemistry The Study Of Change Problem Categories Jordi Salvador Vasquez Academia Edu

Calculate H O From Oh Youtube
How To Calculate H3o And Oh Sciencing
Solved Calculate Either H3o Or Oh For Each Of The Solutions Course Hero

Which Of The Following Statements Is Incorrect

How To Calculate H3o And Oh Sciencing

Acid Base Chemistry Ppt Download

How Do You Calculate The Ph Of A Solution When Given The Oh Concentration Socratic
Solved Calculate Either H3o Or Oh For Each Of The Solutions Course Hero

Concentration Of Acids And Bases Is Usually Described In Molarity Ppt Download
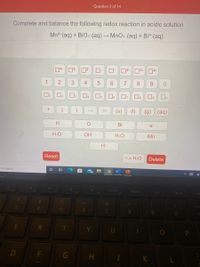
Answered Complete And Balance The Following Bartleby

Derive A Relation Between Ph And Poh